Article 22: TITANIUM APPLICATIONS
Titanium offers
unique opportunities for many industrial applications. Among the highlights
are:
![]() Elevated
strength-to-density ratio
Elevated
strength-to-density ratio
![]() Low
density – roughly half the weight of steel, nickel and copper alloys – which translates
into significantly improved rotordynamics of the
titanium rotors
Low
density – roughly half the weight of steel, nickel and copper alloys – which translates
into significantly improved rotordynamics of the
titanium rotors
![]() Exceptional
corrosion resistance – superior for chlorides, seawater, sour and oxidizing
acidic media
Exceptional
corrosion resistance – superior for chlorides, seawater, sour and oxidizing
acidic media
![]() Excellent
elevated temperature properties (up to 600 0F)
Excellent
elevated temperature properties (up to 600 0F)
![]() Excellent
properties at low temperature. ASME allows usage of Titanium Grade 1 and Grade
2 to – (minus) 75 0F
Excellent
properties at low temperature. ASME allows usage of Titanium Grade 1 and Grade
2 to – (minus) 75 0F
In the past, a more wider applicational opportunities
of titanium have been restricted primarily due to high cost. However, advances
in technology and manufacturing processes have now made significant strides in
reducing the cost, thus making titanium an excellent material of choice of
numerous applications.
When comparing
chemical resistance of metal alloys, four following metals are usually stand out of the crowd:
316SS
/ C-20
/ Hastelloy /
Titanium
According to the chemical resistance guide(a),
Titanium outperforms (the only
one with an “A” rating) all the
others above for such chemicals as:
(Note (a): Fybroc Chemical Resistance Guide, Application Data)
Adiptic Acid, Ammonium Bicarbonate, Ammonium Carbonite, Ammonium Citrate, Aniline, Arsenic Acid, Arsenous Acid, Barium Sulfide, Butyl Benzyl Phthalate, Butylene Glycol, Calcium Carbonate, Calcium Hypochlorite, Calcium Nitrate, Calcium Sulfate, Calcium
Sulfite, Chlorine Dioxide, Chlorine Water, Chloroacetic
Acid, Chloroform, Cobalt Citrate, Dichloroethylene, Diphenyl Oxide, Ferric Chloride, Ferrous Chloride,
Fertilizer Urea Ammonium Nitrate Composition, Gluconic
Acid, Hydriodic Acid, Hydrobromic
Acid, Iron Plating Solution, Lithium Carbonate, Lithium Hypochlorite,
Magnesium Bisulfite, Magnesium Phosphate, Methyl
Ethyl Ketone (MEK), Methyl Isobutyl Ketone (MIBK), Nickel Nitrate, Perchloric
Acid, Peroxide Bleach, Potassium Bicarbonate, Potassium Bromide, Potassium
Dichromate, Potassium Ferricyanide, Potassium
Permanganate, Potassium Sulfate, Propionic Acid,
Propylene Glycol, Silver Nitrate, Sodium Bromide, Sodium Chlorate, Sodium
Chloride, Sodium Hypochlorite, Styrene Acrylic
Emulsion, Sulfur for Fungicides, Tartaric Acid, Titanium Tetrochloride,
Urea.
And for the following, Titanium is the only choice (others
rated as outright unacceptable):
Bromine Wet Gas, Concentrated Ferric Chloride, Wet Gas
Hydrogen Bromide, Sodium Chlorite, Uran Fertilizer Urea Ammonium Nitrite Composition, Urea
Ammonia Water Nitrate.
Machinery Handbook (23rd Edition, page 607),
states: “Titanium is outstanding in its resistance
to strongly oxidizing acids, aqueous chloride solutions, moist chlorine gas,
sodium hypochlorite, and seawater and brine
solutions. Its uses in aircraft engine compressors and in airframe structures
are based on both its high corrosion resistance and high strength to weight
ratio”.
“Materials Selection for Hydrocarbon and Chemical Plant” (David Hansen,
Robert Puyear) says: “Titanium (melting point: 3034 F
(1668 C)). Titanium is a reactive, refractory metal. The most common use of
Titanium and its alloys in the hydrocarbon and chemical process industries is
in heat transfer applications. It is resistant
to a wide range of range of both organic and inorganic corrodents.
It finds relatively widespread acceptance as heat exchanger tubing for
corrosive processes on one side and corrosive cooling water such as seawater on
the other side. Titanium is also commonly used for wet chlorine and for
concentrated hot caustic solutions. Titanium usage is becoming more common as
it becomes more cost competitive with conventional corrosion resistant alloys
such as the 300-series stainless steels. Titanium can be useful in mildly
reducing applications such as wet alkaline sour overhead condensing systems, if
they are properly designed and fabricated. However, titanium (and the other
reactive and refractory metals) can be unstable in strongly reducing
environments. Selection should be based on experience or should be justified by
a testing program. Titanium can become unstable in the presence of powerful
oxidizers. Examples include dry chlorine, red fuming nitric acid and liquid
oxygen. In addition, titanium can be embrittled by
the formation of hydrides”
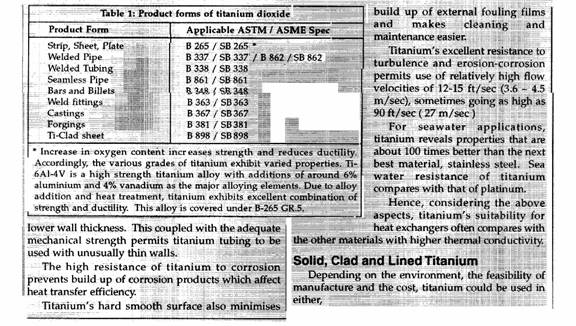
A zero corrosion
allowance can be specified for titanium, resulting in lower wall thickness. This, coupled with the adequate mechanical strength permits
titanium tubing to be used with unusually thin walls.
Corrosion resistance
is not the only strength. Titanium also has the extraordinary erosion
resistance, about 20 times better then Cu-Ni alloys.
Coefficient of
thermal expansion is also low – almost half of stainless steel.
You may also enjoy an
interesting article that was published in Chemical Industry Digest recently,
which is shown below:




Let us know if you may have any questions. We would be glad to help. E-Mail your questions to: